Z Meaning Chemistry
In an uncharged atom the atomic number is also equal to the number of electrons. In chemistry and physics the atomic number of a chemical element also known as its proton number is the number of protons found in the nucleus of an atom of that element and therefore identical to the charge number of the nucleus.
Effective Nuclear Charge Chemistry Tutorial Youtube
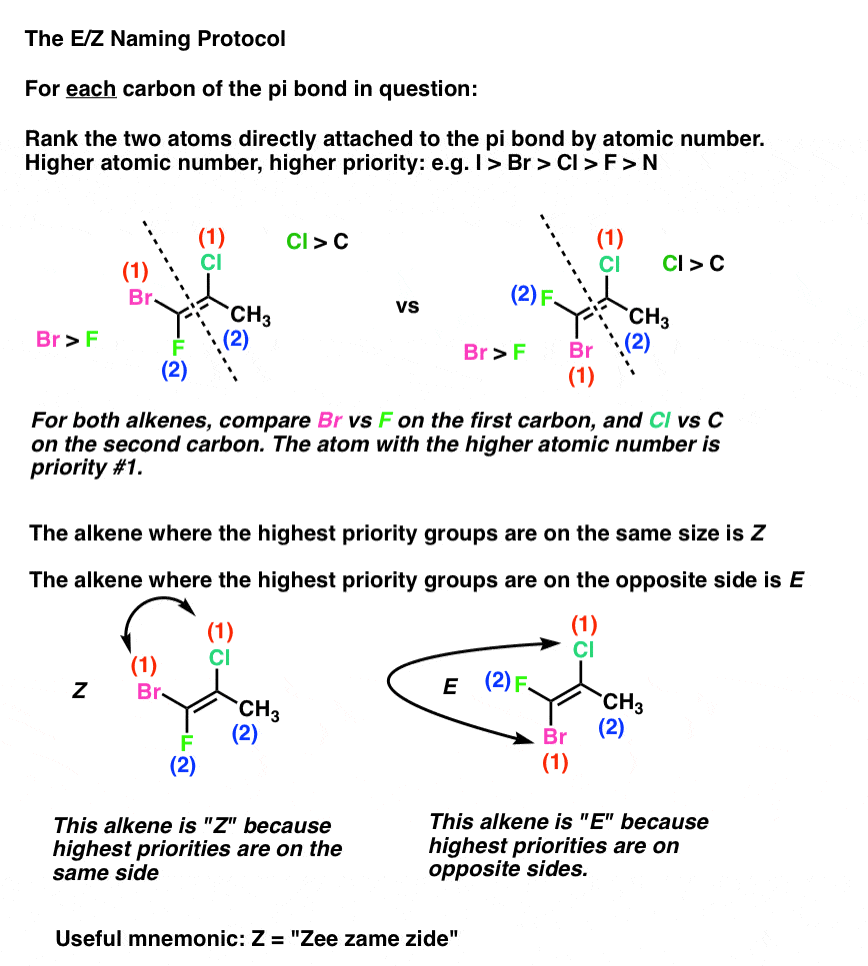
For example an alkene such as but-2-ene has two different groups on each alkene carbon.
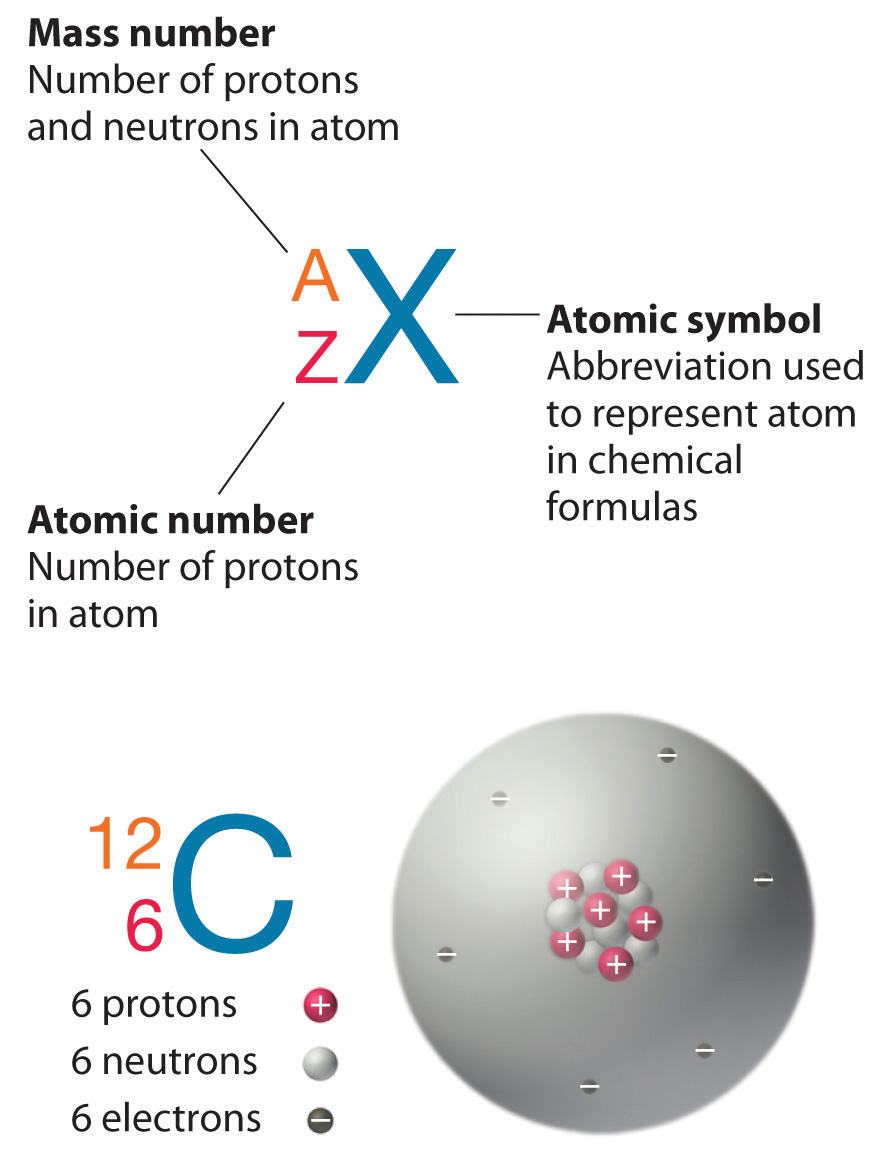
Z meaning chemistry. Ground state - the lowest energy state of an atom ion molecule or subatomic particle. The symbol for impedance. Consider a neutral neon atom Ne a sodium cation Na and a fluorine anion F.
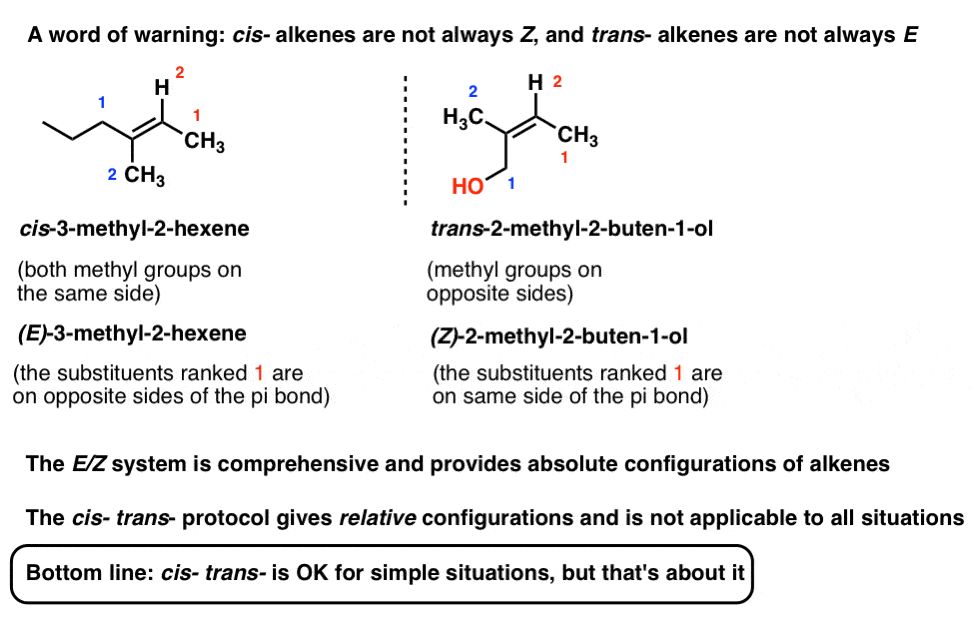
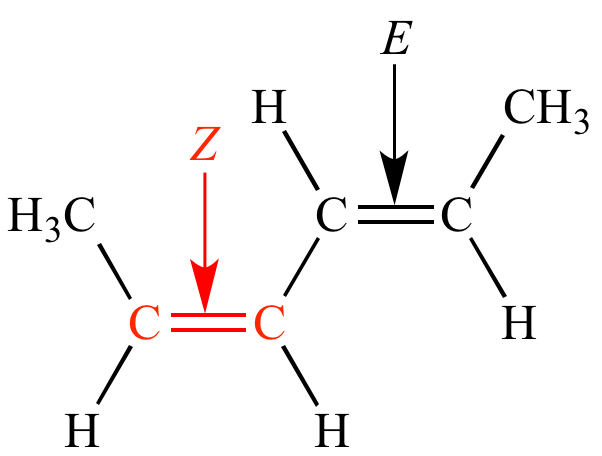
Zs or Zs also zs or Zs 1. However that is not a rule. In simple cases such as 2-butene Z corresponds to cis and E to trans.
The atomic number uniquely identifies a chemical element. Search the Dictionary for More Terms. Illustrated Glossary of Organic Chemistry - Z.
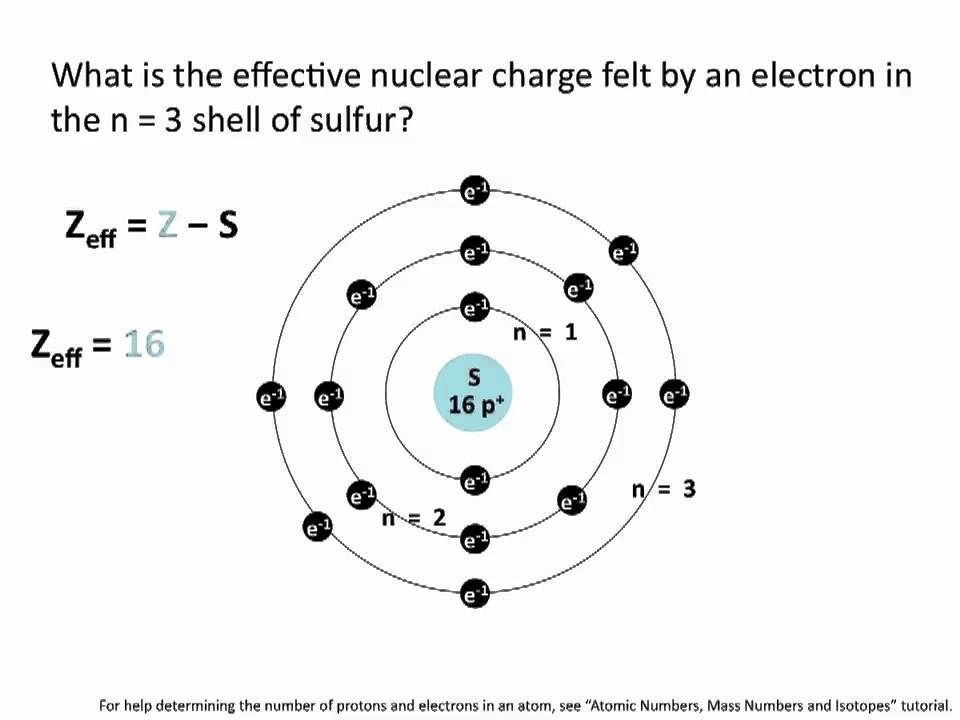
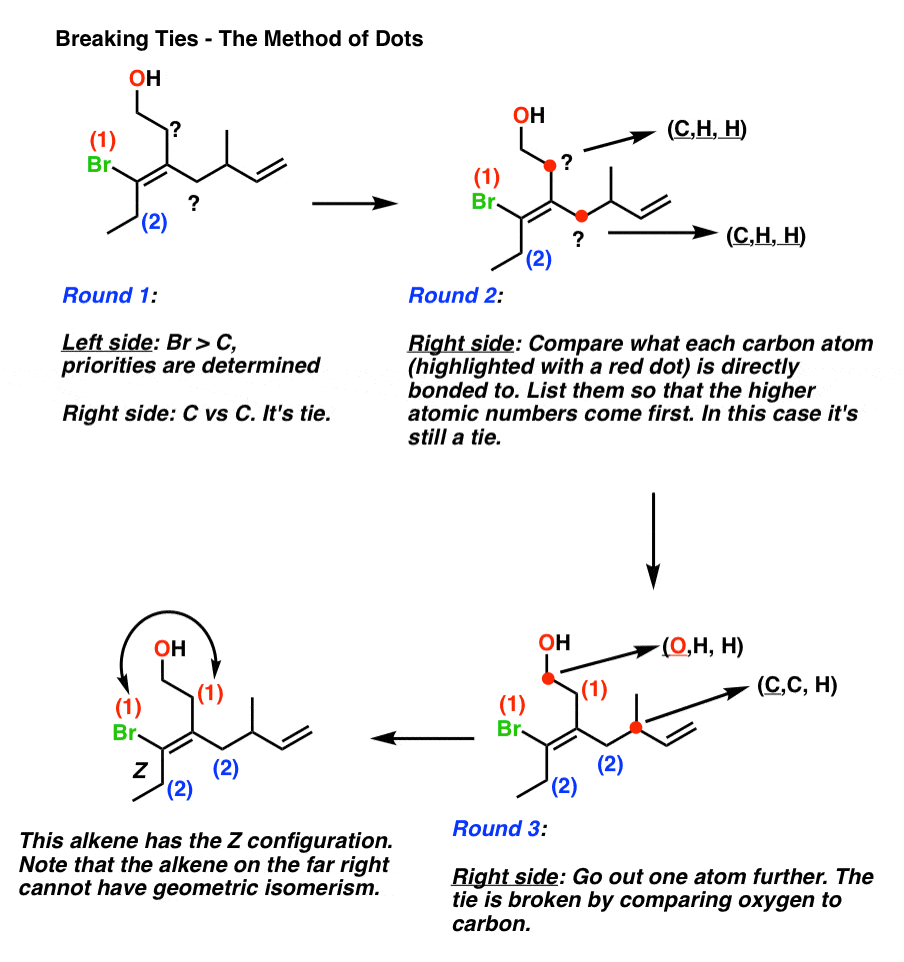
Where Z is the number of protons in the nucleus atomic number and S is the number of electrons between the nucleus and the electron in question the number of nonvalence electrons. Green chemistry - branch of chemistry concerned with lessening the environmental effect of chemicals including development of new materials and processes. If the two groups with the higher priorities are on the same side of the double bond that is described as the Z- isomer.
So you would write it as Z-name of compound. The atomic number uniquely identifies a chemical element. Chemistry chem atomic number.
The single most important characteristic of an atom is its atomic number usually denoted by the letter Z which is defined as the number of units of positive charge protons in the nucleus. Definition of z - Chemistry Dictionary. The symbol Z comes from a German word zusammen which means together.
In E Z isomers you must have. Describes the configurationof a double bondin which the two groupsof highest Cahn-Ingold-Prelog priorityare cis. It can exist as E Z isomers that differ in the positions of the substituents on the double-bonded atoms.
It is identical to the charge number of the nucleus. Illustrated Glossary of Organic Chemistry. In mass spectrometry the ratio of an ions mass m in atomic mass units amu to its formal charge z.
The symbol for atomic number. It is conventionally represented by the symbol Z. General Physics physics impedance.
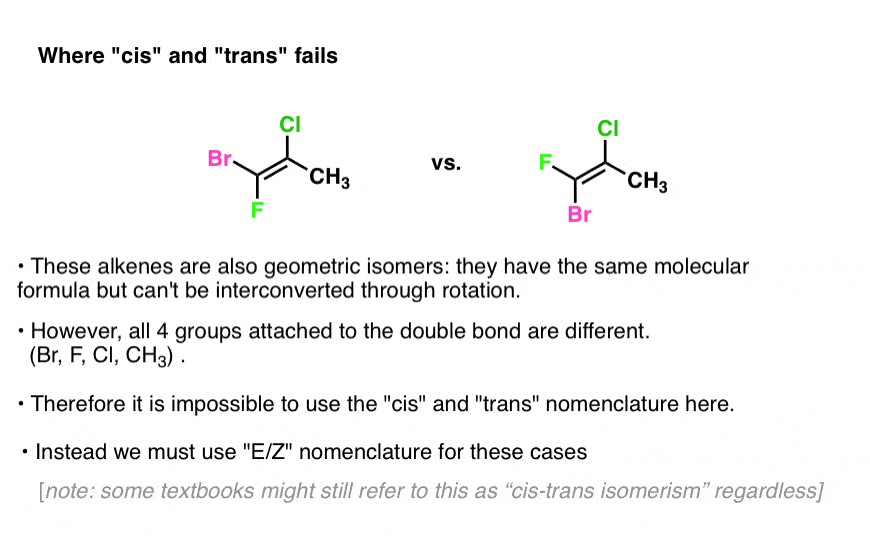
This section and the following one illustrate some idiosyncrasies that happen when you try to compare the two systems. From the German zusammen meaning together. The 26th letter of the modern English alphabet.
For example if an atom has a Z of 6 it is carbon while a Z of 92 corresponds to uranium. The atomic number or proton number symbol Z of a chemical element is the number of protons found in the nucleus of every atom of that element. Z synonyms Z pronunciation Z translation English dictionary definition of Z.
Illustrated Glossary of Organic Chemistry mz mass-to-charge ratio. Two different groups on one end of the bond and two different groups on the other end.
E And Z Notation For Alkenes Cis Trans Master Organic Chemistry
E And Z Notation For Alkenes Cis Trans Master Organic Chemistry

E Z Isomerism Cahn Inglod Prelog Priority Rules Examples Explained Drawings Diagrams Difference In Physical Chemical Properties Cis Trans Isomers For Given Formula A Level Gce As A2 Organic Chemistry Revision Notes
E And Z Notation For Alkenes Cis Trans Master Organic Chemistry

E Z Isomerism Cahn Inglod Prelog Priority Rules Examples Explained Drawings Diagrams Difference In Physical Chemical Properties Cis Trans Isomers For Given Formula A Level Gce As A2 Organic Chemistry Revision Notes
Illustrated Glossary Of Organic Chemistry Z
E Z System Of Nomenclature Of Geometrical Isomers Biyani Group Of Colleges
E And Z Notation For Alkenes Cis Trans Master Organic Chemistry
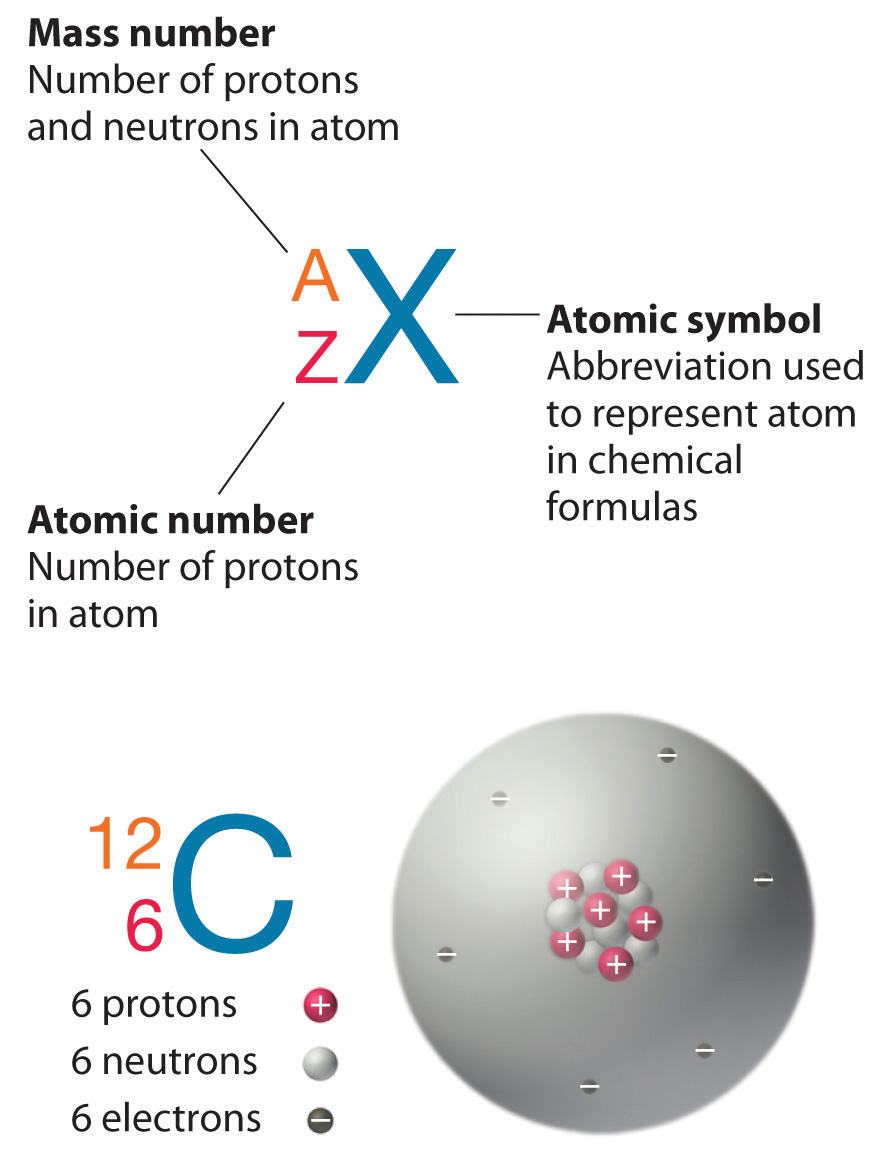
2 2 Atomic Number Mass Number And Atomic Mass Unit Chemistry Libretexts
Post a Comment for "Z Meaning Chemistry"